One of the most interesting and omnipresent states of matter in the kitchen is that of a gel. They have typically the density of a liquid and yet they behave like a solid. That is because in a gel a liquid and a solid are indeed superposed: we get two states of matter for the prize of one.
On the one hand, there is a net formed of long molecules similar to ribbons that link to each other in certain places. On the other, superpose to this network there is a liquid that flows throw it:
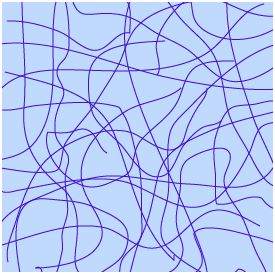
Sketch of gel structure
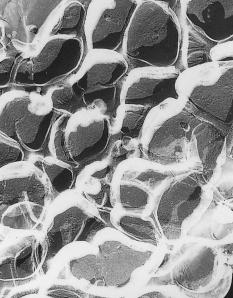
In most gels that liquid is water-based and they can contain as much as 90% of water. Here is a picture (taken with a transmission-electron microscope) of a gel:
An interesting property that most gels have is “thixotropy”: their viscosity decreases the longer they undergo shear stress. For example, a gel is liquid when you agitate it inside the bottle but recovers its gel consistency while at rest.
This is similar to what happens to toothpaste and ketchup: when you squeeze the tube, toothpaste comes out of it but then retains its form on the toothbrush and the same with ketchup. It is not exactly the same property because, although they also decrease their viscosity when undergoing shear stress, this doesn’t depend on the duration of this applied stress but rather on its strength.
Denatured proteins
In the kitchen, the long molecules that form the net are proteins. However, usually proteins are curled up forming a ball, so one needs to stretch them up before they can form the links that give rise to the net. We say that the protein has to be denatured.
Denaturing proteins
Most proteins are denatured at temperatures around 40ºC (104F), others can be unfolded by fast motion like when we whisk an egg, by adding salt (cured meats) or acid (pickling) and also by kneading.
As it turns out, denatured proteins are more digestible that in their initial form. This is because they are much more vulnerable to attack by protein-breaking enzymes and is why we say that cured meats and pickles are somehow “cooked”. Some proteins, such as the collagen in meat and fish, are so tough and stiff before unfolding that they are almost inedible. The reason for collagen to be specially stiff is because it is not only one ribbon but rather a triple helical structure of ribbons (similar to the structure of DNA). To untwist these ribbons collagen needs to be heated about 70ºC (158F).
Forming the net
To have a gel we need not only to have denatured molecules as our ribbons, but we also need them to link to each other forming a net. In the case of collagen such links are formed when cooled down under 15ºC, but if you heat it up again they will break down returning to the liquid phase. Such gels are called thermo-reversible. This is what happens when you put stew, or fish with a sauce in the fridge. The collagen ribbons that have denaturalized from the meat to the sauce form a net when cooled down giving rise to a gel. If you heat it up in the microwave, the sauce goes liquid again.
Egg proteins are an example of a gel which is not thermo-reversible. When the proteins in the egg unfold at temperatures above 40ºC they form chemical bonds between them giving rise to a gel (we say that the egg coagulates). Such links are permanent and stay after the mix is cooled down so that we can enjoy our pudding :).
So every time you are making an omelet, baking pudding, putting stew in the fridge or preparing jelly that you are witnessing a beautiful liquid to gel phase transition.